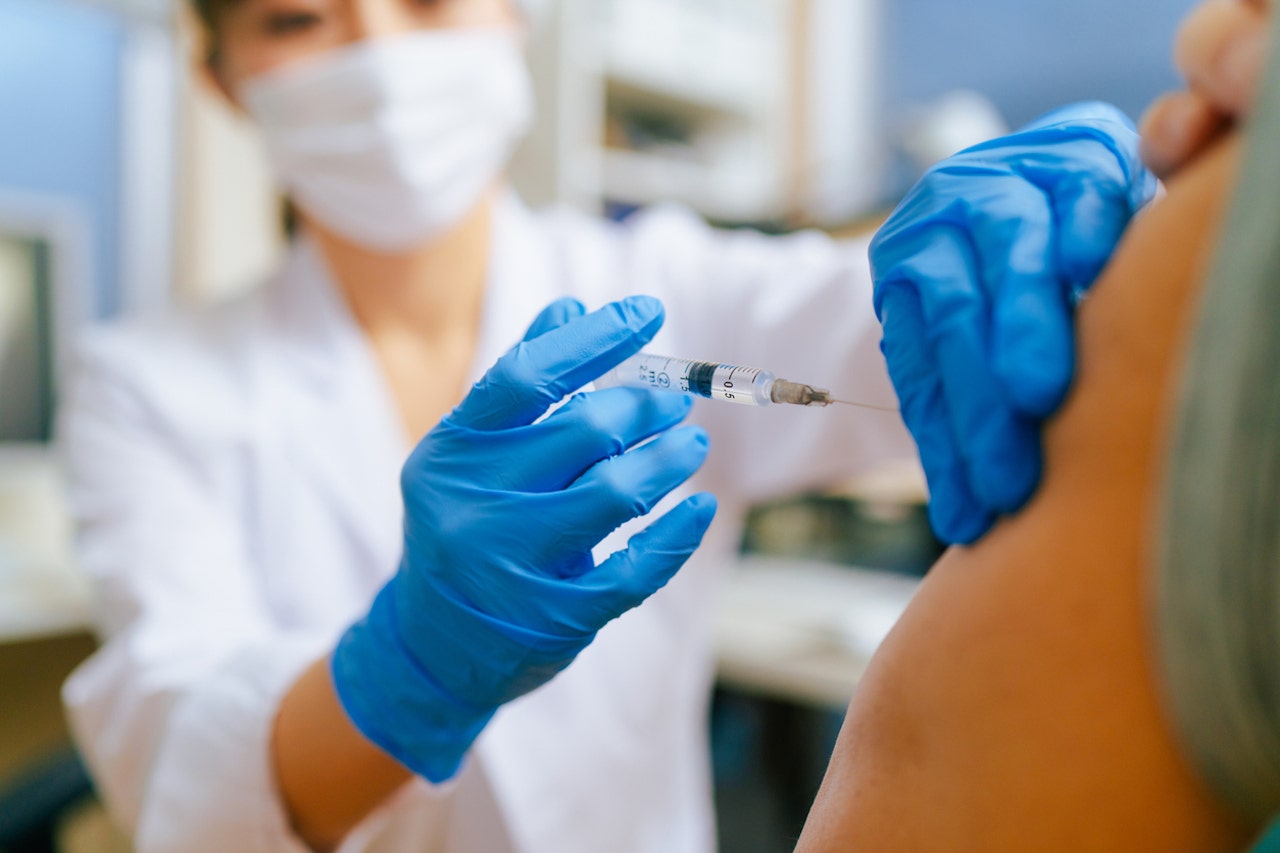
The U.S. Food and Drug Administration (FDA) has approved updated COVID-19 vaccines from Moderna and Pfizer for the 2024-2025 season.
The updated mRNA vaccines, Comirnaty and Spikevax, were fully approved for people 12 years and older, while the Moderna COVID-19 vaccine and Pfizer-BioNTech COVID-19 vaccine were granted emergency authorization for children 6 months through 11 years of age, according to an FDA announcement released today.
The monovalent (single) vaccines are designed to target the Omicron variant KP.2 strain of SARS-CoV-2.
RISKS ASSOCIATED WITH THE COVID VACCINE IDENTIFIED IN STUDY
“These vaccines were updated to provide better protection against COVID-19 caused by circulating variants,” the FDA stated.
“Vaccination continues to be the cornerstone of COVID-19 prevention,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, in the FDA announcement.

The U.S. Food and Drug Administration has approved updated COVID-19 vaccines from Moderna and Pfizer for the 2024-2025 season. (REUTERS/Andrew Kelly/File Photo)
“These updated vaccines meet the agency’s rigorous scientific standards for safety, effectiveness and manufacturing quality.”
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“Given waning immunity of the population from previous exposure to the virus and from prior vaccination, we strongly encourage those who are eligible to consider receiving an updated COVID-19 vaccine to provide better protection against currently circulating variants.”
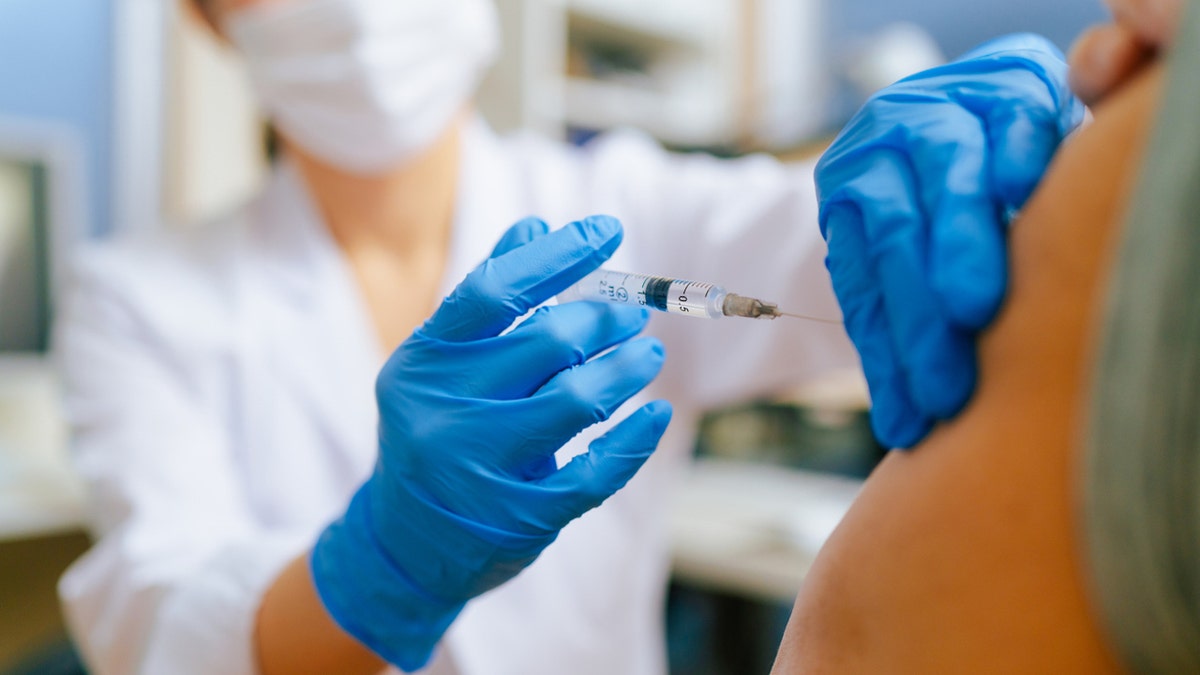
The updated mRNA vaccines were “approved and authorized for emergency use,” according to an FDA announcement. (iStock)
The CDC recommends that everyone 6 months of age and older receives the updated COVID-19 vaccination.
CLICK HERE TO GET THE FOX NEWS APP
That includes women who are pregnant or breastfeeding.
As of the week ending Aug. 10, the Centers for Disease Control and Prevention (CDC) reported that 18.1% of COVID tests were positive.
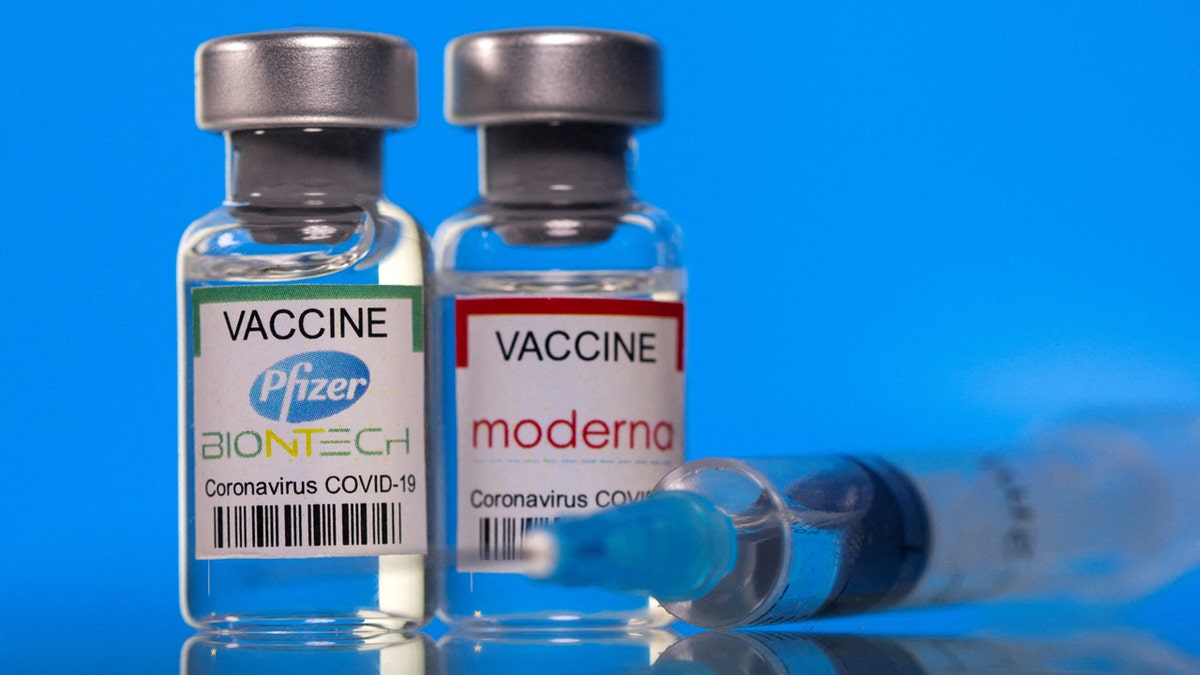
The monovalent (single) vaccines are designed to target the Omicron variant KP.2 strain of SARS-CoV-2. (Reuters/Dado Ruvic/Illustration/File Photo)
Meanwhile, 2.4% of those visiting emergency departments were diagnosed as COVID-19 — a drop of 1.5% from the prior week.
The percentage of deaths related to COVID was 1.9%, per the CDC, up from 1.6% the prior week.
For more Health articles, visit www.foxnews/health
Individuals should speak with their doctor if they have questions about the COVID-19 vaccine, the agency stated.